Microorganisms in the genus Mycobacteria and Nocardia have complex cell walls with higher lipid content. The cell wall of these microbes contains mycolic acid, which is a complex hydrocarbon. Due to this waxy cell wall, bacteria don’t take up the stain easily. Therefore, microorganisms with a waxy cell wall are difficult to stain with routine staining methods such as gram staining or simple staining. To visualize such bacteria special staining method such as Ziehl Neelsen acid fast stain (ZN Stain) is very useful.
The Ziehl-Neelsen (ZN) staining technique is a differential staining technique. First, it was developed by Ziehl and later modified by Neelsen, therefore, this staining technique is named as Ziehl-Neelsen staining. Since we use heat in this procedure, the Ziehl-Neelsen Staining technique is also known as Hot Method of Acid-Fast Staining
The primary goal of this staining method is to distinguish bacteria into acid-fast and non-acid-fast groups.
Z N Stain Principle:
Mycolic acids in cell wall makes the bacterial cell surface waxy. Staining such waxy cell surfaces with aqueous-based stains like the Gram stain is difficult as mycolic acids don’t allow stain penetration.
In ZN staining the smear is first stained with primary stain Carbol Fuchsin. Moderate heat allows the stain to penetrate in the waxy cell wall surface.
Once the Carbol Fuchsin binds to mycolic acids in the cell wall it is not removed even with a strong decolorizer such as an acid alcohol solution.
This property of bacterial cell wall to retain primary stain Carbol fuchsin even after washing with a strong decolorizer (Acid in alcohol Solution) is known as acid fastness. Such bacteria are acid-fast and take up the red color of Crabol Fuschin.
In the next step, the smear is counterstained with secondary dye methylene blue. The bacteria which are decolorized with acid alcohol solution are non-acid fast in nature. only such non-acid fast bacteria take up counterstain methylene blue and appear blue in color.
Reagents for ZN Staining:
The main reagents in ZN staining are Carbol fuchsin, acid alcohol solution, and methylene blue.
Carbol fuchsin:
It is the primary stain. Most species of bacterial cells are stainable with standard aqueous stains like methylene blue and crystal violet. Similarly, mycobacteria are not stainable with common dyes like methylene blue and crystal violet. These bacteria are stainable with carbol fuchsin, a dark red stain prepared in 5% phenol. Heat enhances the penetration of carbol fuchsin through the lipoidal waxy cell wall.
Carbol fuchsin preparation requires distilled water, basic fuchsin, ethyl alcohol, and phenol crystals.
Distilled water- 100ml
Basic fuschin- 1g
Ethyl alcohol (100% ethanol)- 10ml
Phenol crystals- 5ml
2. Acid alcohol solution:
Acid alcohol acts as a decolorizer, i.e., It removes the stain bound to cell surface.
Decolorizer Acid alcohol Solution preparation: (3 percent HCl in 95 percent ethyl alcohol)
Ethyl alcohol- 95 ml
Distilled water- 2 ml
Concentrated hydrochloric acid- 3 ml
3. Methylene blue:
It is the secondary stain which acts as counterstain.
Counterstain Methylene blue preparation:
0.25% methylene blue in 1% acetic acid
Methylene blue- 0.25g
Distilled water- 99ml
Acetic acid- 1ml
Materials required for ZN staining
1. Bacterial culture / sputum sample
2. Clean glass slide
3. Spirit lamp
4. ZN stain reagents
5. Microscope with 100x objective
6. Cedarwood oil or liquid paraffin oil
ZN Staining Procedure:

Image Source: Elizabeth Gray.
Smear Preparation:
1. Take a grease-free and clean glass slide.
2. Aseptically make a smear on the glass slide using the sputum sample or bacterial culture
3. Quickly pass the slide with the smear through the flame for 2-3 times and heat fix the smear or air dry.
Ziehl Neelsen Staining:
1. Flood the smear with primary stain carbon fuchsin. Keep the slide on steam for stain penetration. wait for 5 minutes
2. Allow the slide to cool and wash with tap water.
3. Now flood the smear with decolorizer (Acid alcohol) Solution until the alcohol runs almost clear.
4. Wash the smear on the slide with tap water.
5. Now counterstain the smear with methylene For about 2 minutes.
6. Wash the smear with tap water
7. Blot dry the slide and examine under oil immersion.
Results and Observation :
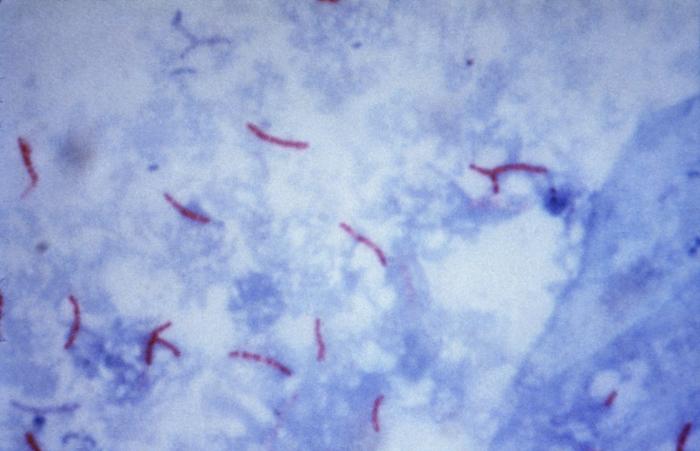
Figure: Mycobacterium tuberculosis visualization using the Ziehl–Neelsen stain. Image Source: CDC/Dr. George P. Kubica.
Acid-fast organisms, Mycobacterium spp., will appear pink-red colored
Nonacid-fast organisms will appear blue.
In addition, background material should stain blue because of methylene blue
Z N Stain Uses:
1. To examine, identify, and classify different Mycobacterium species.
2. To distinguish between acid-fast and non-acid-fast bacilli.
3. For identification of some fungal species, such as Cryptosporidium.
4. The main use of Z N stain is for the diagnosis of pulmonary tuberculosis from sputum samples of patient.

NOTE,WELL PRESENTED
I need my question mark..
I need my questions mark.